Axsome Shares Jump 25% on Alzheimer's Study Results
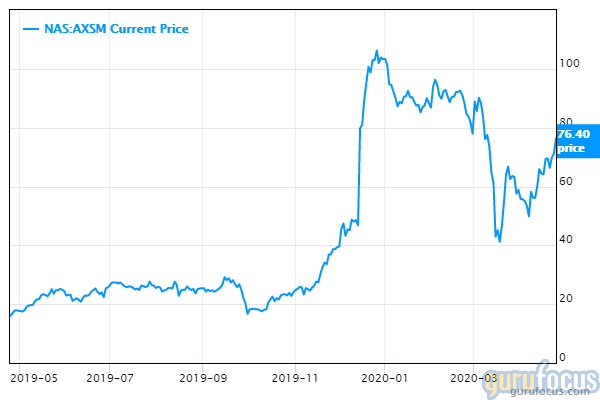
An experimental drug could help caregivers as well as patients, as it substantially improved agitation in Alzheimer's patients in a late-stage clinical trial. The announcement boosted the shares of the maker of the treatment, Axsome Therapeutics (AXSM), nearly 25% to more than $95.
The New York City-based biotech company said it plans to discuss the results of its study of the medication, designated AX-05, with the U.S. Food and Drug Administration, according to an article in BioPharma Dive.
About 70% of Alzheimer's patients experience agitation, which is associated with faster cognitive decline, earlier placement in a nursing home and increased risk of death, according to Axsome. For caregivers, agitation can be a nightmare, as they have to cope with the patient behavior that is all too often harmful or aggressive.
Not only did AX-05 reduce agitation, it was demonstrated to be safe and well tolerated, according to Jeffrey Cummings (M.D., Sc.D., Director Emeritus of the Cleveland Clinic Lou Ruvo Center for Brain Health and Chambers Professor of Brain Science at the University of Nevada Las Vegas).
There is currently no approved treatment for agitation in Alzheimer's disease in the U.S. Patients with moderate-to-severe agitation are usually treated with drugs approved for other conditions. Often, these are antipsychotic drugs, which have been linked to a higher risk of death in dementia, or antidepressants and anxiety medications. All three drug classes have side effect issues, including drowsiness.
Given the need, it's no surprise that a number of pharma companies are developing Alzheimer's treatments, including Eli Lilly and Co. (LLY), Roche (RHHBY), AC Immune (ACIU), MorphoSys (MOR) and Easai (ESALY).
The effectiveness of AX-05 doesn't appear to be limited to aggression in Alzheimer's. Later this year, Axsome plans to ask the FDA for approval to use the drug to treat major depressive disorder. It is also being studied in helping people stop smoking.
Regarding Axsome's other drugs, the company plans to file a new drug application for its migraine medication later this year. It is also testing treatments for narcolepsy and fibromyalgia.
Despite the pop the stock enjoyed Monday, Axsome is still trading below its 52-week high of more than $109. Prior to the announcement of AX-05's success in Alzheimer's aggression, the nine analysts offering 12-month forecasts set a median price target of $125, with a high of $200 and a low of $95, according to CNN Money.
Disclosure: The author holds a position in Eli Lilly
Read more here:
Not a Premium Member of GuruFocus? Sign up for a free 7-day trial here.
This article first appeared on GuruFocus.

 Yahoo Finanzen
Yahoo Finanzen 